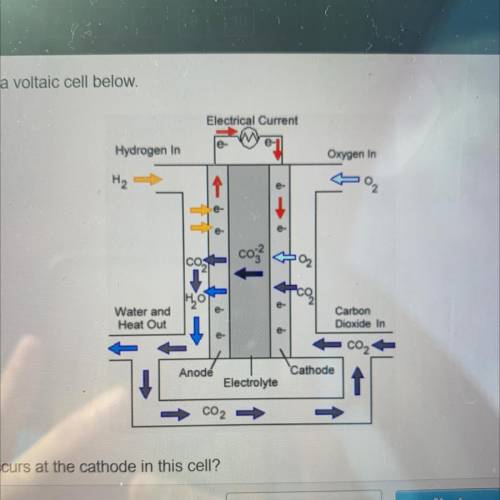
Which half reaction occurs at the cathode in this cell?
2003--O2 + 2002 + 4e-
+ 2002 + 4e--&g...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Questions

Mathematics, 30.01.2020 06:54


Mathematics, 30.01.2020 06:55


History, 30.01.2020 06:55

Computers and Technology, 30.01.2020 06:55

Mathematics, 30.01.2020 06:55

History, 30.01.2020 06:55

Mathematics, 30.01.2020 06:55


Mathematics, 30.01.2020 06:55





Social Studies, 30.01.2020 06:55

Mathematics, 30.01.2020 06:55