
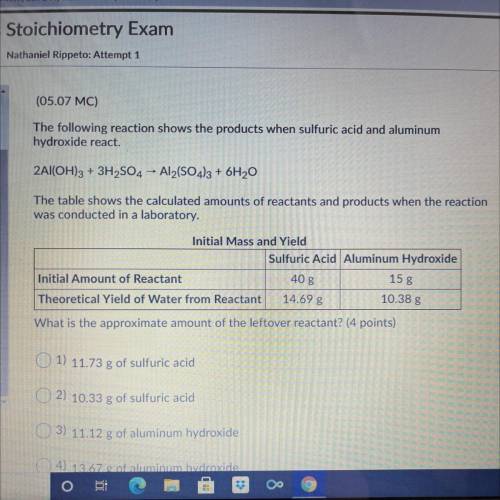
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2Al(OH)3 + 3H2SO4 - Al2(SO4)3 + 6H20
The table shows the calculated amounts of reactants and products when the reaction
was conducted in a laboratory.
What is the approximate amount of the leftover reactant?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2A...
2A...
Questions








Mathematics, 30.05.2020 23:58









Biology, 30.05.2020 23:58





