
Chemistry, 17.02.2021 21:40 emblemhacks
A. Calculate the percentage yield for SiO2 for trial 1. Also, determine the leftover reactant for the trial. Show your Work. b. Based on the percentage yield in trial 2, explain what ratio of reactants is more efficient for the given reaction.
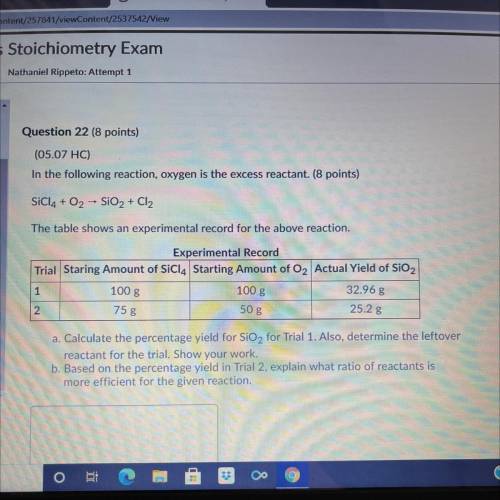

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1



Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
A. Calculate the percentage yield for SiO2 for trial 1. Also, determine the leftover reactant for th...
Questions



English, 02.03.2020 21:19











Health, 02.03.2020 21:19

Mathematics, 02.03.2020 21:19


History, 02.03.2020 21:19


English, 02.03.2020 21:19

Health, 02.03.2020 21:19



