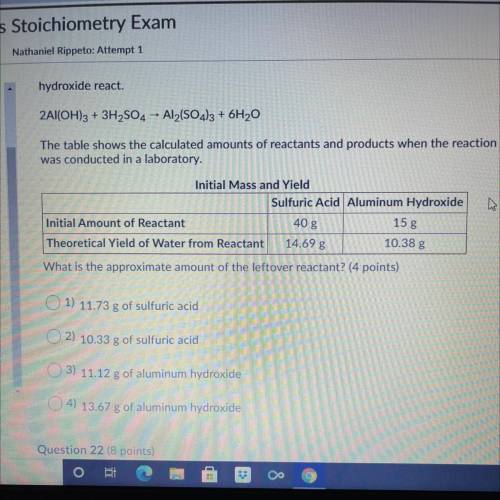
hydroxide react.


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
hydroxide react.
Questions




Mathematics, 18.02.2021 16:40



Mathematics, 18.02.2021 16:40


Biology, 18.02.2021 16:40

Biology, 18.02.2021 16:40

Spanish, 18.02.2021 16:40

Mathematics, 18.02.2021 16:40

Chemistry, 18.02.2021 16:40

Mathematics, 18.02.2021 16:40



Biology, 18.02.2021 16:40

Social Studies, 18.02.2021 16:40


Health, 18.02.2021 16:40