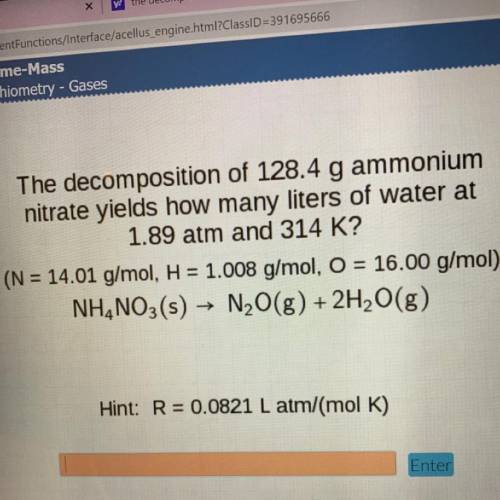
The decomposition of 128.4 g ammonium
nitrate yields how many liters of water at
1.89 atm and...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2


Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1
You know the right answer?
Questions

French, 30.07.2019 02:30





English, 30.07.2019 02:30

Business, 30.07.2019 02:30

Social Studies, 30.07.2019 02:30


Social Studies, 30.07.2019 02:30


History, 30.07.2019 02:30

History, 30.07.2019 02:30


Biology, 30.07.2019 02:30