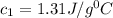Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

You know the right answer?
4 OT 5
A 35g chunk of metal at 130C was dropped in a bucket containing 220g of water at 25C. The fi...
Questions

Mathematics, 07.02.2022 16:30




Mathematics, 07.02.2022 16:30





Mathematics, 07.02.2022 16:40


Arts, 07.02.2022 16:40

Mathematics, 07.02.2022 16:40


Mathematics, 07.02.2022 16:40



Mathematics, 07.02.2022 16:40


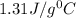
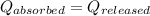
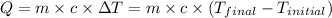
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/1127/3997/b0e58.png)
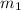 = mass of metal = 35 g
= mass of metal = 35 g
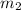 = mass of water = 220 g
= mass of water = 220 g
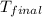 = final temperature =
= final temperature = 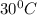
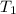 = temperature of metal =
= temperature of metal = 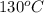
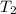 = temperature of water =
= temperature of water = 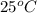
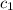 = specific heat of metal = ?
= specific heat of metal = ?
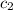 = specific heat of water =
= specific heat of water = 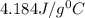
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1127/3997/09236.png)
![35\times c_1\times (30-130)^0C=-[220g\times 4.184\times (30-25)]](/tpl/images/1127/3997/3cabe.png)