Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

You know the right answer?
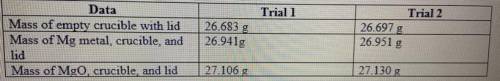
Magnesium is the limiting reactant in this experiment: Calculate the theoretical yield of MgO for ea...
Questions

Mathematics, 20.11.2019 01:31


History, 20.11.2019 01:31

History, 20.11.2019 01:31



Mathematics, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31

Mathematics, 20.11.2019 01:31


Mathematics, 20.11.2019 01:31

History, 20.11.2019 01:31

Chemistry, 20.11.2019 01:31

Health, 20.11.2019 01:31





English, 20.11.2019 01:31

Advanced Placement (AP), 20.11.2019 01:31