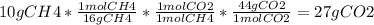Chemistry, 18.02.2021 23:30 aletadaboss
20 POINTS When methane, CH4, is combusted, and produces carbon dioxide, CO2, according to the unbalanced equation CH4 +02 —>CO2 + H2O Write the balanced equation for this reaction, and explain how it is possible for 10 grams of methane fuel to burn in and emit 27 grams of carbon dioxide. Discuss whether or not this reaction obeys the law of conservation of mass.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
20 POINTS When methane, CH4, is combusted, and produces carbon dioxide, CO2, according to the unbala...
Questions

History, 06.05.2021 17:30

English, 06.05.2021 17:30


Mathematics, 06.05.2021 17:30



Mathematics, 06.05.2021 17:30



Mathematics, 06.05.2021 17:30

Advanced Placement (AP), 06.05.2021 17:30




Mathematics, 06.05.2021 17:30

Mathematics, 06.05.2021 17:30

Social Studies, 06.05.2021 17:30

Biology, 06.05.2021 17:30


Social Studies, 06.05.2021 17:30