
Chemistry, 19.02.2021 04:40 Starzdaze5572
Household hydrogen peroxide is an aqueous solution of hydrogen peroxide (H2O2), and its concentration is often measured as a percent by weight. Most drug stores sell 3% solution, which means there are 3.0 g of H2O2 per every 100 g total of solution (H2O2 H2O). For the kinetics experiment, 15 mL of 3% (w/w) hydrogen peroxide solution was poured into a measuring cup with 120 mL of water. 5 mL of 1M sodium carbonate solution (Na2CO3) was also added to the mixture. What is the molar concentration (molarity) of hydrogen peroxide (in mol/L) of the final solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
Household hydrogen peroxide is an aqueous solution of hydrogen peroxide (H2O2), and its concentratio...
Questions

Mathematics, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10


Mathematics, 15.01.2021 20:10



Mathematics, 15.01.2021 20:10




Engineering, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10


Mathematics, 15.01.2021 20:10


Mathematics, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10


Computers and Technology, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10

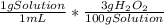 = 0.45 g H₂O₂
= 0.45 g H₂O₂

