
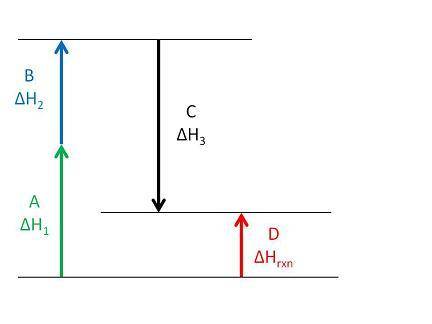
According to the enthalpy diagram below, which of the following statements is true?
Arrows A and D represent the enthalpy of the intermediate chemical reactions.
Arrow C represents the enthalpy of the overall chemical reaction.
Arrow C indicates that the third intermediate reaction is exothermic.
Arrow B indicates that the second intermediate reaction is exothermic.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
According to the enthalpy diagram below, which of the following statements is true?
Arrows A and D...
Questions

Chemistry, 20.09.2021 09:20

Mathematics, 20.09.2021 09:20



Physics, 20.09.2021 09:20

Business, 20.09.2021 09:20

Mathematics, 20.09.2021 09:20

Mathematics, 20.09.2021 09:20

Physics, 20.09.2021 09:20


English, 20.09.2021 09:20


Mathematics, 20.09.2021 09:20


Mathematics, 20.09.2021 09:20


English, 20.09.2021 09:20

Computers and Technology, 20.09.2021 09:20

Mathematics, 20.09.2021 09:20




