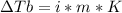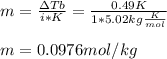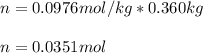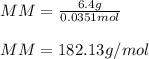Chemistry, 19.02.2021 09:30 marlandwilliams10
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0.49 K higher than the boiling point of pure CC14. Calculate the molar mass of
adrenaline. (K = 5.02 kg K/mol).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
2. The boiling point of a solution containing 6.4 g of the hormone adrenaline in 360 g of
CCl4 is 0...
Questions






Mathematics, 14.04.2020 21:17

History, 14.04.2020 21:17

Advanced Placement (AP), 14.04.2020 21:17


Mathematics, 14.04.2020 21:17




Biology, 14.04.2020 21:18


Mathematics, 14.04.2020 21:18

History, 14.04.2020 21:18