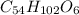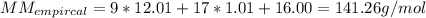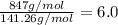Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

You know the right answer?
A fat is composed, in part, of long chains of carbon and hydrogen. This fat as a molecular
mass of...
Questions




History, 31.01.2020 16:45


Health, 31.01.2020 16:45

English, 31.01.2020 16:45

Mathematics, 31.01.2020 16:46


History, 31.01.2020 16:46

History, 31.01.2020 16:46

Mathematics, 31.01.2020 16:46

Health, 31.01.2020 16:46



English, 31.01.2020 16:46