
Chemistry, 19.02.2021 18:50 Jsmooth8928
1 pt
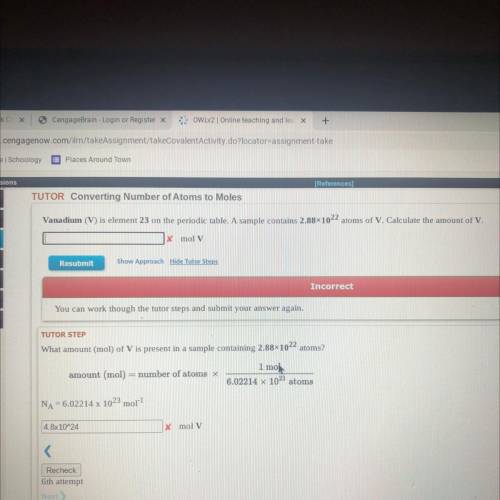
Vanadium (V) is element 23 on the periodic table. A sample contains 2.88x1022 atoms of V. Calculate the amount of V.
1 pt
x mol V
1 pt
Resubmit
Show Approach Hide Tutor Steps

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
You know the right answer?
1 pt
Vanadium (V) is element 23 on the periodic table. A sample contains 2.88x1022 atoms of V. Calc...
Questions

Mathematics, 30.07.2019 15:30

Mathematics, 30.07.2019 15:30

Physics, 30.07.2019 15:30





History, 30.07.2019 15:30



Biology, 30.07.2019 15:30



Chemistry, 30.07.2019 15:30


Social Studies, 30.07.2019 15:30






