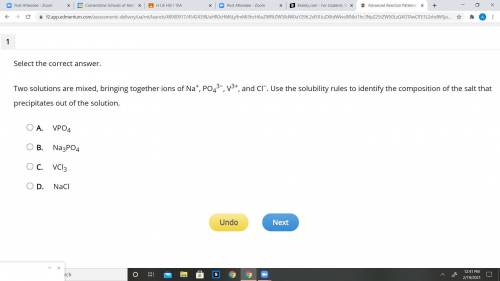
Read the Solubility Rules and answer the Question you see in the pic:
Solubility Rules
Compou...

Chemistry, 19.02.2021 22:00 solivagantjn3010
Read the Solubility Rules and answer the Question you see in the pic:
Solubility Rules
Compounds containing group 1 alkali metals or ammonium (NH4+) are soluble.
Nitrates (NO3−), chlorates (ClO3−), perchlorates (ClO4−), and acetates (C2H3O2−) are soluble.
Chlorides (Cl−), bromides (Br−), and iodides (I−) are soluble, except for compounds containing silver (Ag+), mercury(I) (Hg22+), and lead (Pb2+).
Sulfates (SO42−) are soluble, except for compounds containing calcium (Ca2+), strontium (Sr2+), barium (Ba2+), and lead (Pb2+).
Hydroxides (OH−), carbonates (CO32−), and phosphates (PO43−) are insoluble, except for compounds containing group 1 alkali metals and ammonium (NH4+).
Sulfides (S2−) are insoluble, except for compounds containing group 1 alkali metals, ammonium (NH4+), magnesium (Mg2+), calcium (Ca2+), strontium (Sr2+), and barium (Ba2+).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Questions


Computers and Technology, 23.02.2022 23:50


History, 23.02.2022 23:50


English, 23.02.2022 23:50




Mathematics, 23.02.2022 23:50




Business, 24.02.2022 01:00


Mathematics, 24.02.2022 01:00

Spanish, 24.02.2022 01:00





