Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1


Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
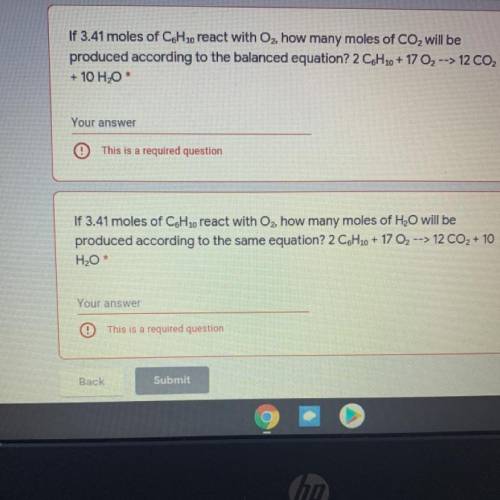
If 3.41 moles of C6H 10 react with Oz, how many moles of CO2 will be
produced according to the bala...
Questions



English, 09.07.2021 09:50


Chemistry, 09.07.2021 09:50

Mathematics, 09.07.2021 09:50

Business, 09.07.2021 09:50

Business, 09.07.2021 09:50

Mathematics, 09.07.2021 09:50


Social Studies, 09.07.2021 09:50


English, 09.07.2021 09:50

Mathematics, 09.07.2021 09:50


Chemistry, 09.07.2021 09:50

Social Studies, 09.07.2021 09:50

English, 09.07.2021 09:50

Social Studies, 09.07.2021 14:00

English, 09.07.2021 14:00