
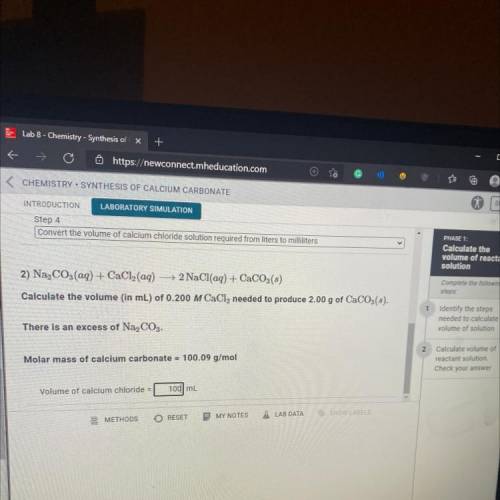
Convert the volume of calcium chloride solution required from liters to milliliters
2) Na, CO3(aq) + CaCl2(aq) + 2 NaCl(aq) + CaCO3(3)
Calculate the volume (in mL) of 0.200 M CaCl, needed to produce 2.00 g of CaCO3(s
There is an excess of Na2CO3.
Molar mass of calcium carbonate = 100.09 g/mol
mL
Volume of calcium chloride =
RESET
MY NOTES
A LAB DATA
METHODS

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
Convert the volume of calcium chloride solution required from liters to milliliters
2) Na, CO3(aq)...
Questions

World Languages, 27.06.2021 14:00

Mathematics, 27.06.2021 14:00

English, 27.06.2021 14:00




Mathematics, 27.06.2021 14:00

Mathematics, 27.06.2021 14:00

Business, 27.06.2021 14:00

English, 27.06.2021 14:00

Mathematics, 27.06.2021 14:00


Mathematics, 27.06.2021 14:00



Mathematics, 27.06.2021 14:00


Mathematics, 27.06.2021 14:00

Mathematics, 27.06.2021 14:00



