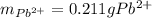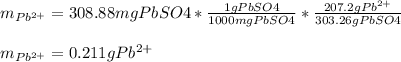Chemistry, 21.02.2021 09:20 hilzepesqtatiana
5 pos. 1. An excess of sodium sulfate was added to a 500. mL sample of polluted water. The
mass of lead (II) sulfate that precipitatcd was 308.88 mg. Determine the mass of lead that was in
the polluted water.
Na2SO4(aq) + Pb2+ (aq) → 2Na(aq) + PbSO4(s)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
5 pos. 1. An excess of sodium sulfate was added to a 500. mL sample of polluted water. The
mass of...
Questions

Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30




Chemistry, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30




Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30

Mathematics, 19.03.2021 20:30