Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 08:30
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
You know the right answer?
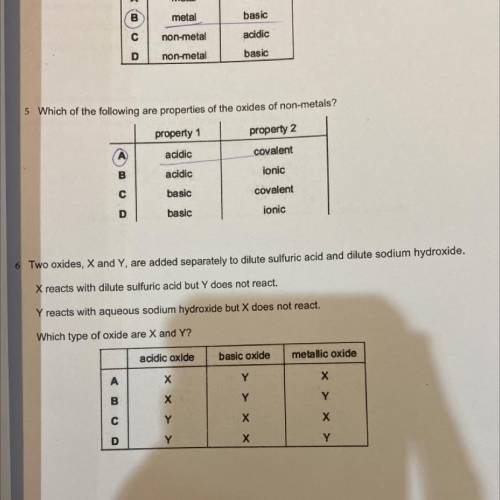
two oxides, X and Y are added seperately to dilute sulfuric acid and dilute sodium hydroxide. X reac...
Questions

Mathematics, 19.01.2021 18:30


English, 19.01.2021 18:30




Mathematics, 19.01.2021 18:30

Mathematics, 19.01.2021 18:30


Health, 19.01.2021 18:30

Mathematics, 19.01.2021 18:30

Mathematics, 19.01.2021 18:30





Mathematics, 19.01.2021 18:30

History, 19.01.2021 18:30