
Chemistry, 21.02.2021 22:20 emmahall0826
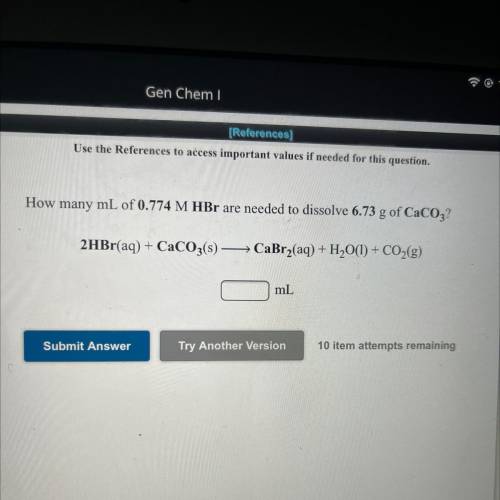
How many mL of 0.774 M HBr are needed to dissolve 6.73 g of CaCO3?
2HBr(aq) + CaCO3(s) -> CaBr2(aq) + H2O(1) + CO2(g)
_mL

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
How many mL of 0.774 M HBr are needed to dissolve 6.73 g of CaCO3?
2HBr(aq) + CaCO3(s) -> CaBr2(...
Questions






Mathematics, 15.12.2019 05:31





History, 15.12.2019 05:31






Mathematics, 15.12.2019 05:31

Mathematics, 15.12.2019 05:31





