
Chemistry, 22.02.2021 01:00 montgomerykarloxc24x
48g of methane was burned in an excess of air. What mass of carbon dioxide would be produced in the reaction assuming complete combustion? Use the information below to answer the question.
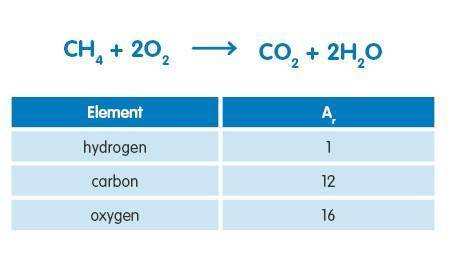

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
48g of methane was burned in an excess of air. What mass of carbon dioxide would be produced in the...
Questions



History, 13.04.2021 06:30


English, 13.04.2021 06:30


English, 13.04.2021 06:30



Mathematics, 13.04.2021 06:30

Mathematics, 13.04.2021 06:30


History, 13.04.2021 06:30


History, 13.04.2021 06:30


History, 13.04.2021 06:30



Mathematics, 13.04.2021 06:30



