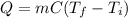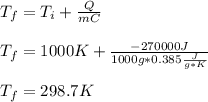Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
A molten sample of 1.00kg of iron with a specific heat of 0.385J/g. K at 1000.K is immersed in a sam...
Questions

Mathematics, 17.11.2019 06:31


Business, 17.11.2019 06:31

Mathematics, 17.11.2019 06:31


Physics, 17.11.2019 06:31

Biology, 17.11.2019 06:31


History, 17.11.2019 06:31

Mathematics, 17.11.2019 06:31

Mathematics, 17.11.2019 06:31


Mathematics, 17.11.2019 06:31

Mathematics, 17.11.2019 06:31


English, 17.11.2019 06:31

Business, 17.11.2019 06:31

Computers and Technology, 17.11.2019 06:31

Biology, 17.11.2019 06:31