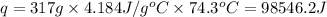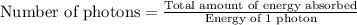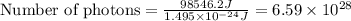Chemistry, 28.12.2019 18:31 stophendless9780
Acontainer with 0.317 l of water is placed into microwave and is then radiated with electromagnetic energy that has a wavelength of 13.3 cm. the temperature of the water then rose by 74.3 °c. calculate the number of photons that were absorbed by the water. assume water has a density of 1.00 g·ml–1 and its specific heat is 4.184 j·g–1·°c–1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Acontainer with 0.317 l of water is placed into microwave and is then radiated with electromagnetic...
Questions





Physics, 25.06.2019 05:30














History, 25.06.2019 05:30

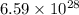
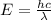
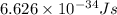
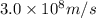
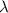 = wavelength of photon = 13.3 cm = 0.133 m (Conversion factor: 1 m = 100 cm )
= wavelength of photon = 13.3 cm = 0.133 m (Conversion factor: 1 m = 100 cm )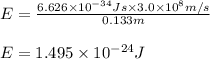
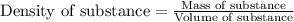
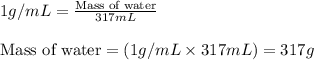
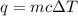
 = change in temperature = 74.3°C
= change in temperature = 74.3°C