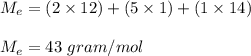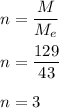Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1


Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
a compound has a molar mass of 129 g/mol if its empirical formula is C2H5N then what is the molecula...
Questions

Chemistry, 20.03.2021 01:10

Mathematics, 20.03.2021 01:10



History, 20.03.2021 01:10


Mathematics, 20.03.2021 01:10

Mathematics, 20.03.2021 01:10


Social Studies, 20.03.2021 01:10


Chemistry, 20.03.2021 01:10


Mathematics, 20.03.2021 01:10



Mathematics, 20.03.2021 01:10

History, 20.03.2021 01:10

Mathematics, 20.03.2021 01:10