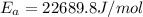Chemistry, 22.02.2021 18:40 danbat3023
The rate constant for a certain reaction is measured at two different temperatures:
temperature k
376.0 °C 4.8 x 10^8
280.0 °C 2.3 x 10^8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy Ea for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3


Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
The rate constant for a certain reaction is measured at two different temperatures:
temperature k
Questions







Chemistry, 05.03.2021 22:00


History, 05.03.2021 22:00


English, 05.03.2021 22:00


History, 05.03.2021 22:00






Mathematics, 05.03.2021 22:00

![ln \frac{k_{2}}{k_{1}} = \frac{-E_{a}}{R}[\frac{1}{T_{2}} - \frac{1}{T_{1}}]](/tpl/images/1136/3217/c502b.png)
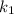 = rate constant at temperature
= rate constant at temperature 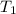 =
= 
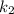 = rate constant at temperature
= rate constant at temperature 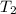 =
= 
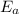 = activation energy = ?
= activation energy = ?
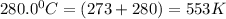
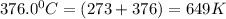
![ln \frac{4.8\times 10^8}{2.3\times 10^8} = \frac{-E_{a}}{8.314}[\frac{1}{649} - \frac{1}{553}]](/tpl/images/1136/3217/fcab8.png)