
Chemistry, 22.02.2021 18:30 lovelybear2354
You will be provided with the AH values for each equation. These values are state dependent,
meaning that liquid water and steam will have different values. You will need to multiply the
AH, value for each compound by the number of moles of that compound in the balanced
chemical equation, as each value of AH, is for 1 mole of compound. Find the enthalpy of
reaction for the following equation:
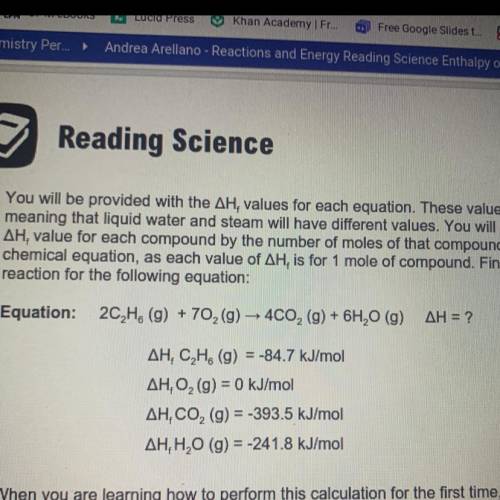

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
You will be provided with the AH values for each equation. These values are state dependent,
meanin...
Questions

Mathematics, 14.03.2020 06:46

English, 14.03.2020 06:46



Mathematics, 14.03.2020 06:46




Mathematics, 14.03.2020 06:47

Mathematics, 14.03.2020 06:48

English, 14.03.2020 06:48




Mathematics, 14.03.2020 06:49

Chemistry, 14.03.2020 06:49

Mathematics, 14.03.2020 06:49



Mathematics, 14.03.2020 06:50



