
Chemistry, 30.09.2019 03:30 ConfusedJuliana
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775 ml of water. calculate the following:
a) moles of k2so4
b)millimoles of k2so4
c)molarity of k2so4, k+, so4(2-)
d)ppm of k2so4
e)%(w/v) k2so4
f)pk+
g)pso4(2-)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

You know the right answer?
a solution was prepared by dissolving 177 mg of potassium sulfate (k2so4, mw = 174.24 g/mol) in 775...
Questions


Mathematics, 22.06.2019 16:40




Mathematics, 22.06.2019 16:40

Spanish, 22.06.2019 16:40


Mathematics, 22.06.2019 16:40


Mathematics, 22.06.2019 16:40

English, 22.06.2019 16:40


History, 22.06.2019 16:40

Mathematics, 22.06.2019 16:40


History, 22.06.2019 16:40

Mathematics, 22.06.2019 16:40


History, 22.06.2019 16:40

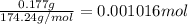
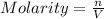
![[K_2SO_4]=\frac{0.001016 mol}{0.775 L}=0.001311 mol/L](/tpl/images/0275/4296/7b54d.png)
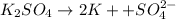
![[K^+]=2\times [K_2SO_4]=2\times 0.001311 mol/L=0.002622 mol/L](/tpl/images/0275/4296/87b7a.png)
![[SO_4^{2-}]=1\times [K_2SO_4]=1\times 0.001311 mol/L=0.001311 mol/L](/tpl/images/0275/4296/fd0e3.png)
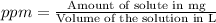
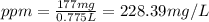
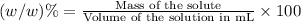
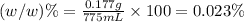
![pK^=-\log[K^+]](/tpl/images/0275/4296/a9ce4.png)
![pK^+=-\log[0.002622 M]=2.58](/tpl/images/0275/4296/0acce.png)
![pSO_4^{2-}=-\log[SO_4^{2-}]](/tpl/images/0275/4296/bd352.png)
![pK^+=-\log[0.001311 M]=2.88](/tpl/images/0275/4296/30697.png)


