
Chemistry, 22.02.2021 19:30 tejalawatson91
Which of the following are correct for zero-order reactions?
A. A higher concentration of reactants will not increase the reaction rate.
B. The units for the rate constant and the rate of reaction are the same.
C. The rate of reaction does not equal the rate constant.
D. The concentration of the reactants changes nonlinearly.
E. A zero-order reaction slows down as the reaction proceeds.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1



Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Which of the following are correct for zero-order reactions?
A. A higher concentration of reactants...
Questions


History, 11.02.2021 07:00


English, 11.02.2021 07:00

Mathematics, 11.02.2021 07:00


Mathematics, 11.02.2021 07:00


Mathematics, 11.02.2021 07:00



Mathematics, 11.02.2021 07:00

Spanish, 11.02.2021 07:00


History, 11.02.2021 07:00

Biology, 11.02.2021 07:00




Mathematics, 11.02.2021 07:00

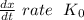 doesn't depend on reaction rate, a higher reaction rate does not intensify the reaction.
doesn't depend on reaction rate, a higher reaction rate does not intensify the reaction.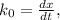 the created based and the reaction rate is about the same.
the created based and the reaction rate is about the same.

