
Chemistry, 23.02.2021 07:20 shelatzcreed
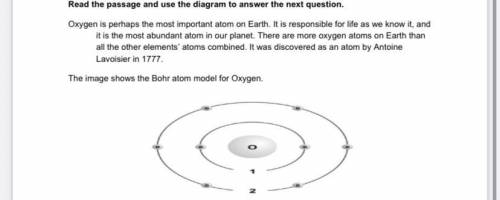
Which of the following electronic transitions in the oxygen atom will result in light emission?
A. ni= 1⟶nf= 2
B. ni= 1⟶nf=3
C. ni =3⟶nf =2
D. ni =2⟶nf =3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Which of the following electronic transitions in the oxygen atom will result in light emission?
A....
Questions

Mathematics, 24.05.2020 00:58


Biology, 24.05.2020 00:58


Computers and Technology, 24.05.2020 00:58



Biology, 24.05.2020 00:58

Physics, 24.05.2020 00:58


Business, 24.05.2020 00:58


English, 24.05.2020 00:58

Mathematics, 24.05.2020 00:58

English, 24.05.2020 00:58


Chemistry, 24.05.2020 00:58

Mathematics, 24.05.2020 00:58

Computers and Technology, 24.05.2020 00:58

Mathematics, 24.05.2020 00:58



