
Chemistry, 23.02.2021 07:20 titofigueroa777
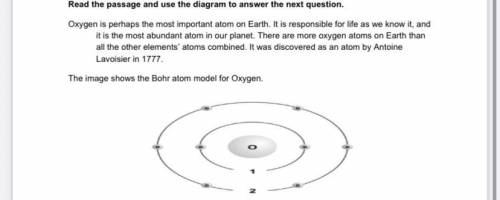
Which of the following electronic transitions in the oxygen atom will result in light emission?
A. n i= 1⟶nf = 2
B. n i= 1⟶nf =3
C. n i =3⟶n f =2
D. n i =2⟶n f =3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Which of the following electronic transitions in the oxygen atom will result in light emission?
A....
Questions

Mathematics, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00


Physics, 05.07.2019 13:00


History, 05.07.2019 13:00

Spanish, 05.07.2019 13:00


Mathematics, 05.07.2019 13:00

Geography, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00


Mathematics, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00

History, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00

Mathematics, 05.07.2019 13:00



