
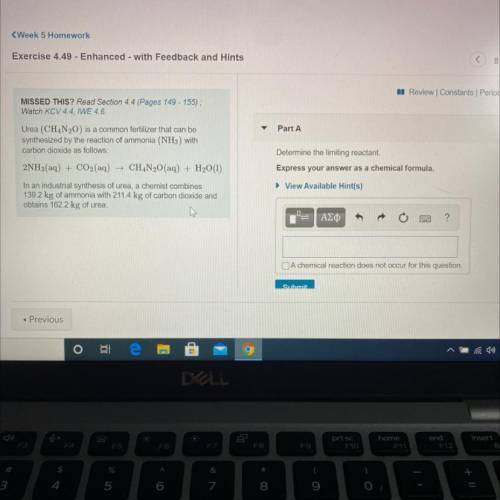
Urea (CH4N20) is a common fertilizer that can be
synthesized by the reaction of ammonia (NH3) with
carbon dioxide as follows:
Determine the limiting reactant.
Express your answer as a chemical formula.
2NH3(aq) + CO2(aq) + CH4N20(aq) + H2O(1)
In an industrial synthesis of urea, a chemist combines
139.2 kg of ammonia with 211.4 kg of carbon dioxide and
obtains 162.2 kg of urea.
Determine the limiting reactant

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2

Chemistry, 23.06.2019 11:30
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Urea (CH4N20) is a common fertilizer that can be
synthesized by the reaction of ammonia (NH3) with...
Questions



Chemistry, 28.12.2019 03:31

Social Studies, 28.12.2019 03:31


Chemistry, 28.12.2019 03:31

English, 28.12.2019 03:31



Biology, 28.12.2019 03:31

Physics, 28.12.2019 03:31

Mathematics, 28.12.2019 03:31

History, 28.12.2019 03:31

Geography, 28.12.2019 03:31

History, 28.12.2019 03:31


Mathematics, 28.12.2019 03:31


Biology, 28.12.2019 03:31

History, 28.12.2019 03:31



