compute the theoretical yield of the product (in

Chemistry, 23.02.2021 09:10 marissagirl9893
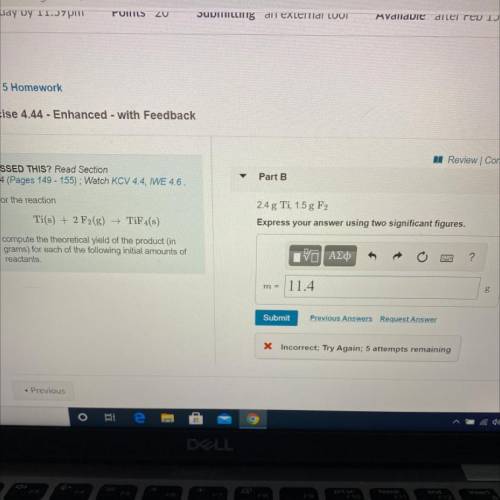
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
grams) for each of the following initial amounts of
reactants.
2.4 g Ti, 1.5 g F2
Express your answer using two significant figures.
please help! will give brainliest.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
compute the theoretical yield of the product (in
Questions

History, 03.02.2021 09:50


Health, 03.02.2021 09:50


Geography, 03.02.2021 09:50


Social Studies, 03.02.2021 14:00


Mathematics, 03.02.2021 14:00


History, 03.02.2021 14:00

Mathematics, 03.02.2021 14:00

English, 03.02.2021 14:00


History, 03.02.2021 14:00


Mathematics, 03.02.2021 14:00

English, 03.02.2021 14:00


Advanced Placement (AP), 03.02.2021 14:00



