
Chemistry, 23.02.2021 14:20 sadieanngraham15
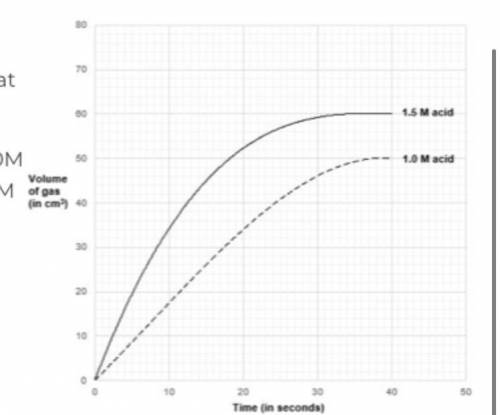
determine the rate of reaction at 10 seconds when : a) concentration of acid is used at 1.0M b) concentration of acid is used at 1.5M

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 11:00
Suppose you increase your walking speed from 7 m/s to 15 m/s in a period of 1 s. what is your acceleration?
Answers: 1
You know the right answer?
determine the rate of reaction at 10 seconds when : a) concentration of acid is used at 1.0M b) conc...
Questions



History, 24.05.2021 02:20

Mathematics, 24.05.2021 02:20





Physics, 24.05.2021 02:20

History, 24.05.2021 02:20

Biology, 24.05.2021 02:20






Mathematics, 24.05.2021 02:20


Physics, 24.05.2021 02:20



