Balance the following equations:
1. __H2 + __N2 → __NH3...

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
Questions


Mathematics, 12.10.2020 07:01




History, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01

Spanish, 12.10.2020 07:01

Chemistry, 12.10.2020 07:01



Mathematics, 12.10.2020 07:01


Health, 12.10.2020 07:01

Mathematics, 12.10.2020 07:01

Geography, 12.10.2020 07:01


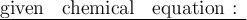

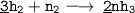
 !!
!!

