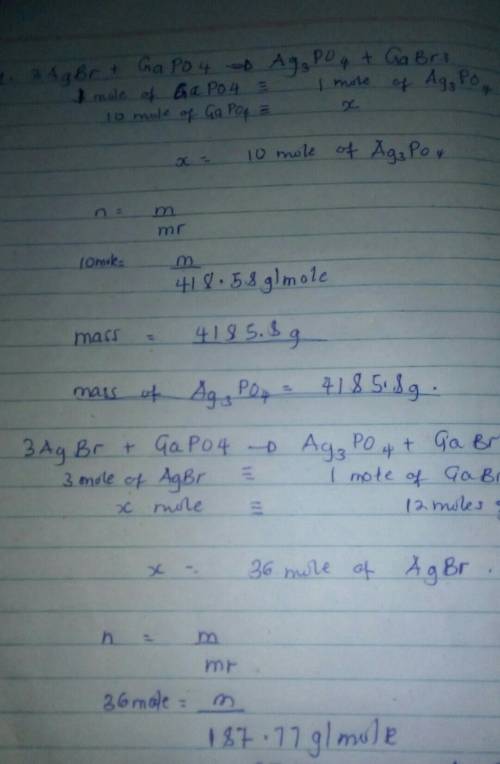Chemistry, 23.02.2021 21:20 aljalloh94
3AgBr + GaPO4 = Ag3PO4 + GaBr3
1. How many grams of Ag3PO4 are produced from the use of 10.0 moles of GaPO4?
2. How many grams of AgBr are needed to produce 12 moles of GaBr3?
3. How many grams of GaBr3 are produced from the reaction of 15 moles of GaPO4?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
3AgBr + GaPO4 = Ag3PO4 + GaBr3
1. How many grams of Ag3PO4 are produced from the use of 10.0 moles...
Questions


Computers and Technology, 05.02.2020 07:00

Mathematics, 05.02.2020 07:00


Social Studies, 05.02.2020 07:00

Mathematics, 05.02.2020 07:00


Mathematics, 05.02.2020 07:00

History, 05.02.2020 07:00

Mathematics, 05.02.2020 07:00







Spanish, 05.02.2020 07:00