
Chemistry, 23.02.2021 21:10 deanlmartin
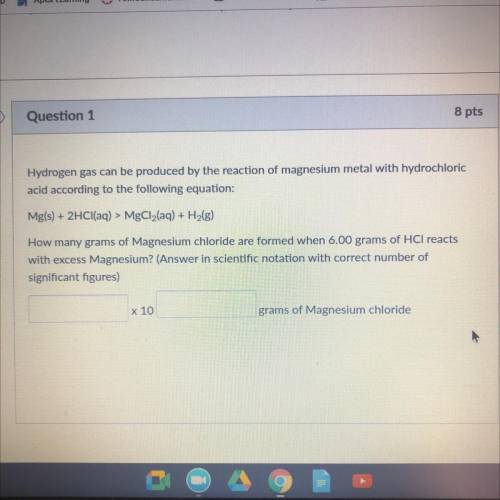
Hydrogen gas can be produced by the reaction of magnesium metal WICH
acid according to the following equation:
Mg(s) + 2HCl(aq) > MgCl2(aq) + H2(g)
How many grams of Magnesium chloride are Irmed when 6.00 grams of HCl reacts
with excess Magnesium? (Answer in scientific notation with correct number of
significant figures)
x 10
grams of Magnesium chloride

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Hydrogen gas can be produced by the reaction of magnesium metal WICH
acid according to the followin...
Questions

Mathematics, 31.10.2020 02:30

Spanish, 31.10.2020 02:30

Mathematics, 31.10.2020 02:30


Mathematics, 31.10.2020 02:30

Mathematics, 31.10.2020 02:30

Spanish, 31.10.2020 02:30


English, 31.10.2020 02:30



History, 31.10.2020 02:30

Mathematics, 31.10.2020 02:30



Arts, 31.10.2020 02:30

Health, 31.10.2020 02:30

Mathematics, 31.10.2020 02:30

English, 31.10.2020 02:30

Mathematics, 31.10.2020 02:30



