
Chemistry, 23.02.2021 22:30 arnold2619
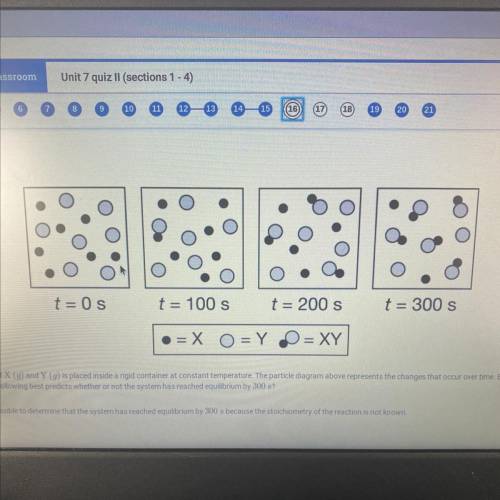
An equimolar mixture of X (g) and Y (g) is placed inside a rigid container at constant temperature. The particle diagram above represents the changes that occur over time. Based on the particle
diagram, which of the following best predicts whether or not the system has reached equilibrium by 300 s?
a. It is not possible to determine that the system has reached equilibrium by 300 s because the stoichiometry of the reaction is not known.
b. it is not possible to determine that the system has reached equilibrium by 300 s because the amounts of X, Y, and XY have continued to change
c. The system has reached equilibrium by 300 s because the rate of formation of XY is constant
d. The system has reached equilibrium by 300 s because the rates of consumption of X and Y are equal

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1


Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
An equimolar mixture of X (g) and Y (g) is placed inside a rigid container at constant temperature....
Questions





Mathematics, 12.03.2020 20:27

Biology, 12.03.2020 20:27




Social Studies, 12.03.2020 20:28

History, 12.03.2020 20:28

Mathematics, 12.03.2020 20:28

Mathematics, 12.03.2020 20:28



Mathematics, 12.03.2020 20:28







