
Chemistry, 24.02.2021 02:00 StupidFatChipmunk
For each of the following acid/base reactions, use curved-arrow notation to show the movement of electrons and to predict the products. Label acids and bases as either Bronsted-Lowery or Lewis. I don't understand how to do either someone please explain.
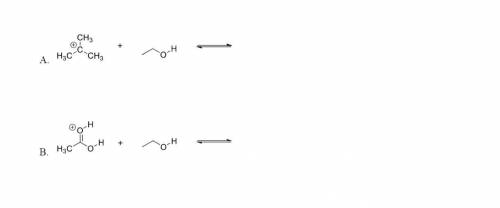

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2


Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
For each of the following acid/base reactions, use curved-arrow notation to show the movement of ele...
Questions

Mathematics, 06.05.2020 02:33




Mathematics, 06.05.2020 02:33


Social Studies, 06.05.2020 02:33


English, 06.05.2020 02:33

Mathematics, 06.05.2020 02:33


History, 06.05.2020 02:33

Mathematics, 06.05.2020 02:33


History, 06.05.2020 02:33


Mathematics, 06.05.2020 02:33





