
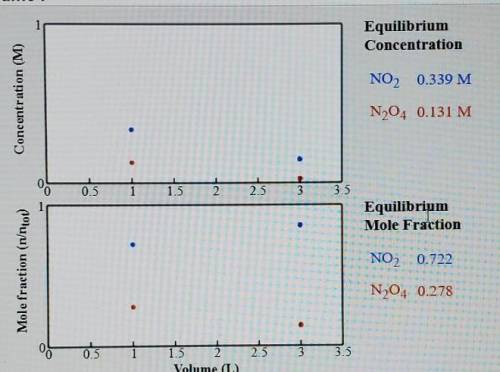
Compare the system (still at 50 C) with a volume of 1.0 L. What is the total amount of gas(mol) present in the container of each of these volumes.
K=0.880 if this is even needed.
I would appreciate just answering the 1.0L with steps so I can work out the second one. Thank you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

You know the right answer?
Compare the system (still at 50 C) with a volume of 1.0 L. What is the total amount of gas(mol) pres...
Questions

Mathematics, 09.11.2019 23:31






Mathematics, 09.11.2019 23:31




Biology, 09.11.2019 23:31



Mathematics, 09.11.2019 23:31

Social Studies, 09.11.2019 23:31

Mathematics, 09.11.2019 23:31


Chemistry, 09.11.2019 23:31

Mathematics, 09.11.2019 23:31



