
Chemistry, 24.02.2021 21:00 familyk0jj3
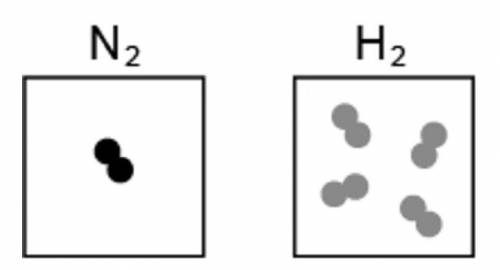
The image represents the reaction between a certain number of molecules of N2 and H2. Two squares are shown. Inside the left square one unit of two joint circles if shown. The molecular formula of nitrogen gas is shown on top of this square. The square on the right has four units of two joint circles. The molecular formula for hydrogen gas is shown on top of this square If the maximum amount of NH3 is formed during the reaction, what is the leftover reactant?
One molecule of N2
One molecule of H2
Two molecules of N2
Two molecules of H2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
The image represents the reaction between a certain number of molecules of N2 and H2. Two squares ar...
Questions

Computers and Technology, 07.10.2021 14:00

Computers and Technology, 07.10.2021 14:00

Mathematics, 07.10.2021 14:00



Business, 07.10.2021 14:00


Mathematics, 07.10.2021 14:00

Biology, 07.10.2021 14:00

Computers and Technology, 07.10.2021 14:00

Physics, 07.10.2021 14:00

Mathematics, 07.10.2021 14:00


Social Studies, 07.10.2021 14:00

Mathematics, 07.10.2021 14:00





Computers and Technology, 07.10.2021 14:00



