
Chemistry, 24.02.2021 21:30 CameronVand21
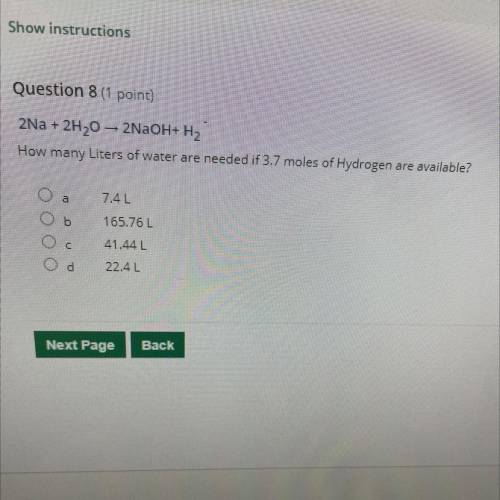
2Na + 2H20 – 2NaOH+H2 How many Liters of water are needed if 3.7 moles of Hydrogen are available?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1


Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
2Na + 2H20 – 2NaOH+H2
How many Liters of water are needed if 3.7 moles of Hydrogen are available?
<...
Questions

History, 30.07.2019 02:50







English, 30.07.2019 03:00





Biology, 30.07.2019 03:00


Social Studies, 30.07.2019 03:00




Social Studies, 30.07.2019 03:00




