Percent of C-14 remaining in sample:
50%
Half-life of C-14:
5,730
Age estimate in...

Chemistry, 24.02.2021 23:40 emilybrown21304
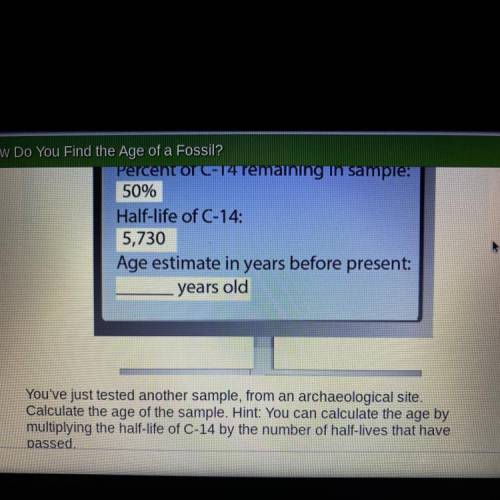
Percent of C-14 remaining in sample:
50%
Half-life of C-14:
5,730
Age estimate in years before present:
years old
You've just tested another sample, from an archaeological site.
Calculate the age of the sample. Hint: You can calculate the age by
multiplying the half-life of C-14 by the number of half-lives that have passed

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
Questions

History, 02.01.2020 20:31

Mathematics, 02.01.2020 20:31

History, 02.01.2020 20:31






Mathematics, 02.01.2020 20:31

English, 02.01.2020 20:31

History, 02.01.2020 20:31




Advanced Placement (AP), 02.01.2020 20:31

Geography, 02.01.2020 20:31




Mathematics, 02.01.2020 20:31



