
Chemistry, 25.02.2021 01:00 bazsinghnagoke
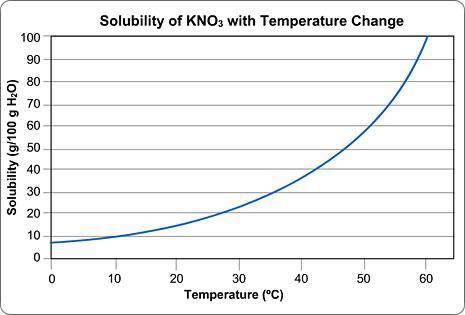
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you've dissolved about 55 g of KNO3, you can't dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of the water?
Round your answer to the nearest whole number and submit the number only; no units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after...
Questions


English, 08.07.2019 06:30

English, 08.07.2019 06:30

Mathematics, 08.07.2019 06:30

Social Studies, 08.07.2019 06:30

Arts, 08.07.2019 06:30

Biology, 08.07.2019 06:30

History, 08.07.2019 06:30

Social Studies, 08.07.2019 06:30

Biology, 08.07.2019 06:30



Chemistry, 08.07.2019 06:30

Biology, 08.07.2019 06:30

Mathematics, 08.07.2019 06:30


Mathematics, 08.07.2019 06:30

Health, 08.07.2019 06:30


Health, 08.07.2019 06:30



