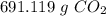Stoichiometry is hawd
Using the following reaction:
C12H22O11 + 12 O2 → 12CO2 + 11H2O +...

Chemistry, 25.02.2021 04:40 starfox5454
Stoichiometry is hawd
Using the following reaction:
C12H22O11 + 12 O2 → 12CO2 + 11H2O + energy
How many grams of carbon dioxide, CO2, are produced from metabolizing 448g sucrose?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Questions



Mathematics, 20.02.2020 09:06



Social Studies, 20.02.2020 09:06



English, 20.02.2020 09:06



Mathematics, 20.02.2020 09:06

English, 20.02.2020 09:07

English, 20.02.2020 09:07

Mathematics, 20.02.2020 09:07


Mathematics, 20.02.2020 09:07


Mathematics, 20.02.2020 09:07

History, 20.02.2020 09:08

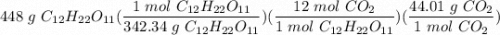 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: