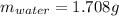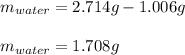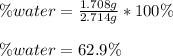A 2.714 gram sample of a hydrate of sodium carbonate is placed in a test
tube and heated until the mass is constant. If the mass of the dry sodium
carbonate is 1.006 grams. What was the mass of water driven off and what
was the percentage of water in the hydrate?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
A 2.714 gram sample of a hydrate of sodium carbonate is placed in a test
tube and heated until the...
Questions

Mathematics, 06.06.2020 00:59

Mathematics, 06.06.2020 00:59

Mathematics, 06.06.2020 00:59

Mathematics, 06.06.2020 00:59


Mathematics, 06.06.2020 00:59


Social Studies, 06.06.2020 00:59



History, 06.06.2020 00:59



English, 06.06.2020 00:59


Mathematics, 06.06.2020 00:59

Mathematics, 06.06.2020 00:59


Mathematics, 06.06.2020 00:59

Chemistry, 06.06.2020 00:59