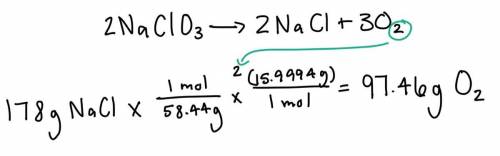Chemistry, 25.02.2021 09:00 komari0217
Given the following equation: 2NaClO3 --> 2NaCl + 3O2 If 178 g of NaCl are produced in this reaction, how many grams of O2 are also produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1
You know the right answer?
Given the following equation: 2NaClO3 --> 2NaCl + 3O2
If 178 g of NaCl are produced in this reac...
Questions

English, 07.12.2019 05:31



Mathematics, 07.12.2019 05:31

Mathematics, 07.12.2019 05:31



Mathematics, 07.12.2019 05:31

Chemistry, 07.12.2019 05:31


Mathematics, 07.12.2019 05:31


Mathematics, 07.12.2019 05:31





Mathematics, 07.12.2019 05:31


Mathematics, 07.12.2019 05:31