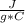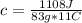Chemistry, 25.02.2021 14:00 kimberlyvazquez1121
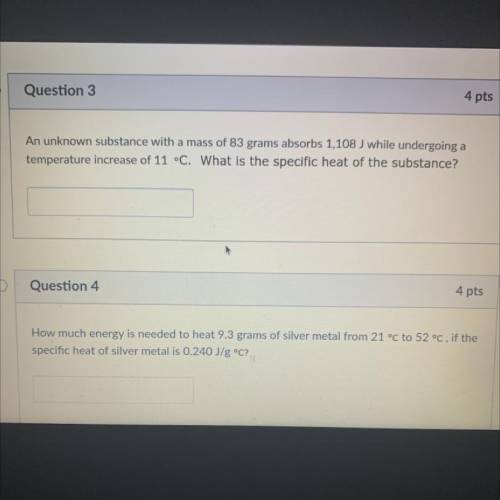
An unknown substance with a mass of 83 grams absorbs 1,108 J while undergoing a
temperature increase of 11 °C. What is the specific heat of the substance?
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1


Chemistry, 23.06.2019 06:50
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1
You know the right answer?
An unknown substance with a mass of 83 grams absorbs 1,108 J while undergoing a
temperature increas...
Questions



Geography, 13.07.2019 12:40


History, 13.07.2019 12:40



English, 13.07.2019 12:40

Geography, 13.07.2019 12:40





History, 13.07.2019 12:40

Spanish, 13.07.2019 12:40


Biology, 13.07.2019 12:40


Social Studies, 13.07.2019 12:40