
Chemistry, 25.02.2021 14:00 Dreambig85
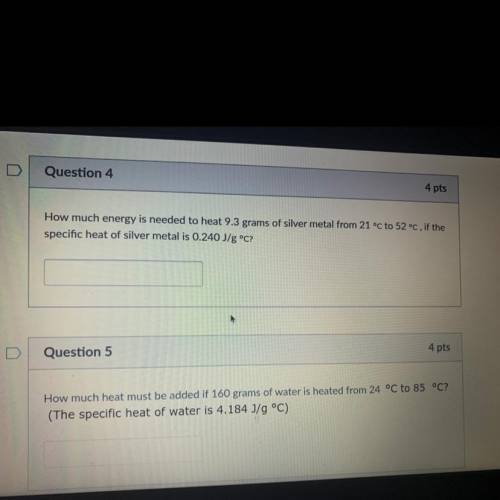
How much energy is needed to heat 9.3 grams of silver metal from 21 °C to 52 °C, if the
specific heat of silver metal is 0.240 J/g °C?
Question 5
4 pts
How much heat must be added if 160 grams of water is heated from 24 °C to 85 °C?
(The specific heat of water is 4.184 J/g °C)
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
How much energy is needed to heat 9.3 grams of silver metal from 21 °C to 52 °C, if the
specific he...
Questions


Mathematics, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57


Mathematics, 06.06.2020 03:57

Mathematics, 06.06.2020 03:57

Social Studies, 06.06.2020 03:57

Physics, 06.06.2020 03:57






Chemistry, 06.06.2020 03:57




English, 06.06.2020 03:57

English, 06.06.2020 03:57



