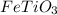Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

You know the right answer?
What is the formula of a compound that has [1 mol Fe], [1 mol Ti], and [3 mol O]...
Questions


Mathematics, 06.10.2019 03:00



Biology, 06.10.2019 03:00



Social Studies, 06.10.2019 03:00


Mathematics, 06.10.2019 03:00


Physics, 06.10.2019 03:00


Mathematics, 06.10.2019 03:00





Mathematics, 06.10.2019 03:00

Biology, 06.10.2019 03:00