
Chemistry, 25.02.2021 18:20 alejandr1872913
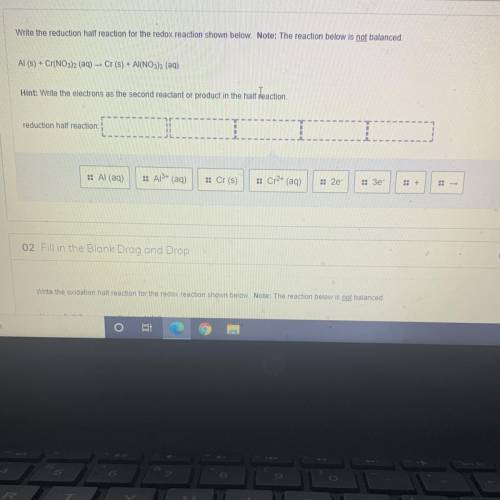
Write the reduction half reaction for the redox reaction shown below. Note: The reaction below is not balanced.
Al(s) + Cr(NO3)2 (aq) – Cr(s) + Al(NO3)3 (aq)
Hint: Write the electrons as the second reactant or product in the half reaction.
reduction half reaction:
:: Al (aq)
:: Al3+ (aq)
:: Cr(s)
:: Cr2+ (aq)
:: 2e
:: Зе-

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

You know the right answer?
Write the reduction half reaction for the redox reaction shown below. Note: The reaction below is no...
Questions

History, 20.11.2020 03:50

English, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Physics, 20.11.2020 03:50



Mathematics, 20.11.2020 03:50

Spanish, 20.11.2020 03:50

Advanced Placement (AP), 20.11.2020 03:50

Mathematics, 20.11.2020 03:50


German, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50

Chemistry, 20.11.2020 03:50

Mathematics, 20.11.2020 03:50


History, 20.11.2020 03:50



