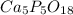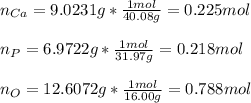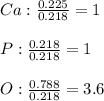Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
A sample of a compound used to polish dentures and as a nutrient and dietary supplement is analyzed...
Questions


Physics, 23.02.2020 03:31

Mathematics, 23.02.2020 03:33

Mathematics, 23.02.2020 03:33


Mathematics, 23.02.2020 03:35

History, 23.02.2020 03:35

Mathematics, 23.02.2020 03:37


History, 23.02.2020 03:38

Mathematics, 23.02.2020 03:39





English, 23.02.2020 03:40


Mathematics, 23.02.2020 03:41